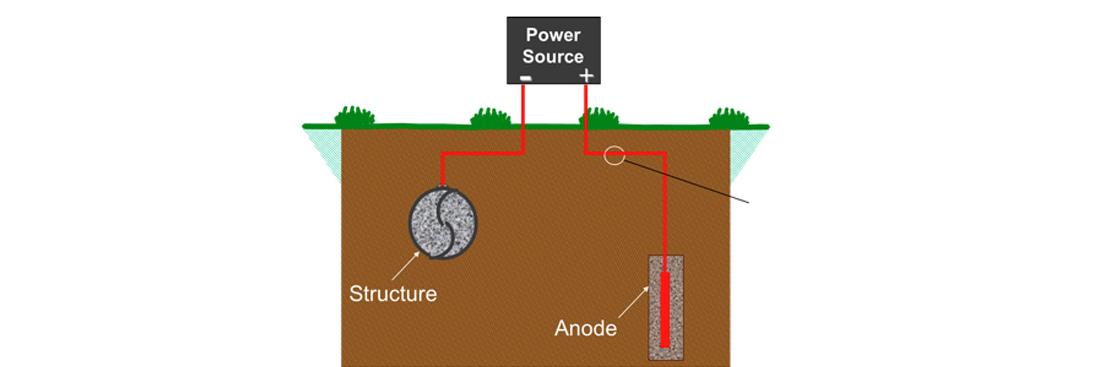
Impressed Current Cathodic Protection System
An impressed current system consists of an external power source (rectifier), anodes, anode backfill, wiring, and connections. The external power source forces current to flow from the anode to the structure through the electrolyte. The anodes used in impressed current cathodic protection systems are different from those used in galvanic systems and are usually constructed of a relatively inert material.
Impressed current anodes are manufactured from materials that are consumed at low rates. They generally operate at higher current and driving voltage levels than galvanic anode systems.
Anodes materials that have been used as impressed current anodes include:
- High-silicon, chromium, cast iron
- Platinum-coated titanium and niobium
- Mixed metal oxide-coated titanium
HIGH SILICON IRON
High-silicon cast iron (HSCI) is a chemically resistant alloy containing silicon, chromium and iron. HSCI anodes are commonly used in fresh water, seawater, or underground applications. Silicon-chromium-iron anodes are brittle but the hardness of the alloy makes the anode less susceptible to damage from abrasion or erosion.
It is supplied with a prepared carbonaceous backfill material for underground application. When installed without backfill and where oxygen evolution is the predominant anodic reaction, the silicon chromium-iron anodes perform better than graphite. In dry soil the silicon dioxide film, which forms on the surface, introduces a high resistance that degrades performance. HSCI is very brittle and forms a SiO² film on the surface in underground applications that can increase the resistance of the anode in dry environments. The consumption rate of HSCI ranges from 0.25 to 1 kg/A-y (0.55 to 2.20 lb/A-y).


PLATINUM / PLATINISED TITANIUM ANODES
The two most common platinized anodes incorporate titanium and niobium substrates. The anodes were developed primarily for use in seawater and other chloride environments since both substrates form protective, dielectric, oxide layers when made anodic in the presence of chlorides. Platinized anodes are economical only when operated at high current densities such as in seawater where there can be rapid migration of chlorides to the anode and products away from the anode. Where the predominant anodic reaction is the evolution of oxygen, higher driving voltages are needed. In terms of both the costs of the anode and the power required, it is therefore not usually economical for underground applications.
Platinum is used as an anode material when either metallurgically clad or plated onto either a titanium or niobium substrate. Titanium and niobium form stable oxide layers when made anodic. The consumption rate of platinized anodes is on the order of 6 to10 mg/A-y.
Platinized anodes are available in wire or mesh form and are most suitable in fresh-or salt-water applications rather than in underground applications.



MIXED METAL OXIDE
Mixed-metal oxide (MMO) anodes (also referred to as DSA for dimensionally stable anode) consist of rare earth oxides (electrocatalytic activated coatings) baked onto a titanium substrate. These anodes were developed for the electrolytic production of chlorine and hypochlorites but are now used for cathodic protection applications.
The metal oxide coating is highly conductive and demonstrates an extremely low weight loss. Mixed metal oxide anodes are extremely resistant to acid attack even at a pH less than one. These anodes are available in rod, tube, or mesh form and are used in fresh water, saltwater and underground environments, as well as embedded in concrete. The consumption rate is on the order of 1 mg/A-y.
Ruthenium MMO coated titnium anodes are typically used in seawater as the evolution of chloride gas is of no concern. Iridium MMO coated anodes are used in coke, freshwater, soil or sand. The titanium substrate remains constant throughout the design life of the anode and is manufactured using titanium which meets ASTM B338 Grade 1 or 2 Standards.
The operating characteristics for MMO coating loadings are shown below:
| Electrolyte | Maximum Design Current Density |
Anode life |
|---|---|---|
| Carbonaceous backfill | 50 A/m² | 20 years |
| Calcined petroleum coke | 100 A/m² | 20 years |
| Fresh water | 100 A/m² | 20 years |
| Brackish water | 100 A/m²- 300 A/m² (#) | 20 years |
| Sea Water | 600 A/m² | 20 years |
# Current density should be determined in accordance with brackish water resistivity.
| Environment | Anode Dimensions (mm) | Anode Current Output | Anode life |
|---|---|---|---|
| Sea Water | Ø19 x 1200 | 45 | 20 years |
| Ø25 x 500 | 25 | 20 years | |
| Ø25 x 1000 | 50 | 20 years | |
| Ø25 x 1200 | 60 | 20 years | |
| Ø25 x 1500 | 75 | 20 years | |
| Ø32 x 1200 | 75 | 20 years |
* Anode current rating can be customised by modifying the length of the anode. Anode life can
be extended up to 30 years or more by increasing the thickness of the MMO coating.
Typical uses of impressed current cathodic protection are:
-
- For large current requirements, particularly for bare or poorly coated structures
- In all electrolyte resistivities
- As an economical way of protecting structures having dissipated galvanic anodes
- To overcome stray current or cathodic interference problems
- For protection of large heat exchanger water boxes, oil heater-treaters, and other vessels
- For interiors of water storage tanks
- For exterior bottoms (both primary and secondary) of above ground storage tanks
- For underground storage tanks
- For underwater components of offshore structures
- For foundation piles and sheet piling,both underground and in the water.
